Irbesartan CAS 138402-11-6 Assay 98.0~102.0% (HPLC) API USP Standard Antihypertensive
Ruifu Chemical is the leading supplier of Irbesartan (CAS: 138402-11-6) with high quality, can meet the USP standard. Ruifu Chemical has been supplying APIs and pharmaceutical intermediates more than 15 years.
Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase Irbesartan and related intermediates, please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | Irbesartan |
| Synonyms | BMS-186295; SR-47436; Aprovel; Avapro; 2-Butyl-3-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one; 2-Butyl-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one |
| CAS Number | 138402-11-6 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C25H28N6O |
| Molecular Weight | 428.54 |
| Density | 1.30±0.10 g/cm3 |
| Melting Point | 184.0 to 188.0℃ |
| Solubility in Water | Insoluble in Water |
| COA & MSDS | Available |
| Storage Temperature | Store Long-Term at 2-8℃ |
| Origin of Product | Shanghai, China |
| Product Categories | API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Test Items | Specifications | Results |
| Appearance | White to almost white crystalline powder | White crystalline powder |
| Identification | ||
| IR | Should comply with reference standard | Complies |
| HPLC | Should comply with reference standard | Complies |
| Water Content (By K.F) | ≤0.50% | 0.15% |
| Sulphated Ash | ≤0.20% | <0.20% |
| Heavy Meatals | ≤0.002% | <0.002% |
| Limit of Azide | ≤10ppm | <10ppm |
| Organic Impurities | ||
| Irbesartan Related Compounds A | ≤0.20% | 0.002% |
| Any Other Individual Impurity | ≤0.10% | 0.004% |
| Total Impurities | ≤0.50% (Excluding R-Isomer) | 0.33% |
| Residual Solvents | ||
| Ethanol | ≤5000 ppm | 28ppm |
| Dichloromethane | ≤600 ppm | Not Detected |
| N,N-Dimethylformamide | ≤880 ppm | Not Detected |
| t-Butyl Methyl Ether | ≤5000 ppm | Not Detected |
| Xylene | ≤1500ppm | 320ppm |
| Toluene | ≤890ppm | Not Detected |
| Assay (HPLC) | 98.0~102.0% of Irbesartan (C25H28N6O), calculated on the anhydrous basis | 99.9% |
| Conclusion | The product has been tested and complies with the USP 36 Standard. | |
Package: Bottle, Aluminium foil bag, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry (2-8℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.

Hazard Symbols Xn - Harmful
Risk Codes 22 - Harmful if swallowed
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S24/25 - Avoid contact with skin and eyes.
WGK Germany 3
RTECS HM2950270
HS Code 2933290090
Irbesartan (CAS: 138402-11-6) is an angiotensin II receptor antagonist used mainly for the treatment of hypertension. Irbesartan was developed by Sanofi Research (now part of Sanofi-Aventis). It is jointly marketed by Sanofi-Aventis and Bristol-Myers Squibb under the trade names Aprovel, Karvea, and Avapro. Irbesartan is used to treat high blood pressure. Avapro was launched in Germany, the UK and the US for hypertension. As with all angiotensin II receptor antagonists, irbesartan is indicated for the treatment of hypertension.
Irbesartan may also delay progression of diabetic nephropathy and is also indicated for the reduction of renal disease progression in patients with type 2 diabetes, hypertension and microalbuminuria (>30 mg/24 hours) or proteinuria. Irbesartan can also reduce electrical remodeling of the myocardium, thereby reduce the mortality rate of patients with hypertension, it is the most effective drug for treatment of hypertension and cardiovascular disease.
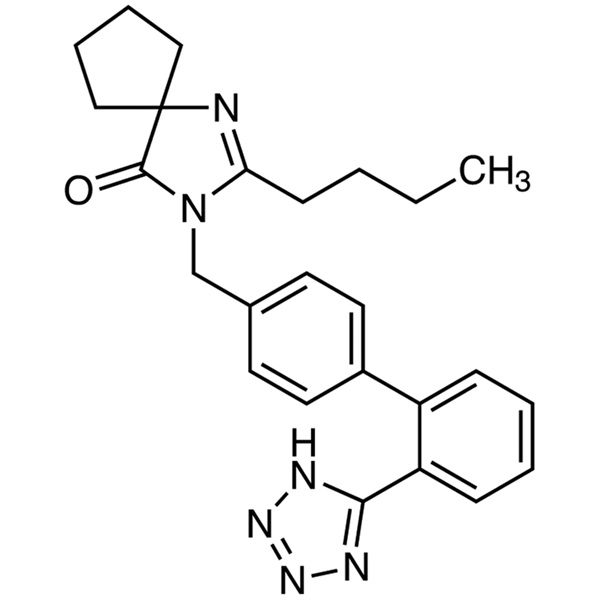
Irbesartan
C25H28N6O 428.53
1,3-Diazaspiro[4.4]non-1-en-4-one, 2-butyl-3-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-;
2-Butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one [138402-11-6]; UNII: J0E2756Z7N.
DEFINITION
Irbesartan contains NLT 98.0% and NMT 102.0% of irbesartan (C25H28N6O), calculated on the anhydrous basis.
IDENTIFICATION
Change to read:
• A. SPECTROSCOPIC IDENTIFICATION TESTS <197>, Infrared Spectroscopy: 197K (CN 1-MAY-2020)
• B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• PROCEDURE
Buffer: Phosphoric acid and water (v/v) (5.5:950). Adjust with triethylamine to a pH of 3.2.
Mobile phase: Acetonitrile and Buffer (330:670)
System suitability solution: 0.05 mg/mL each of USP Irbesartan RS and USP Irbesartan Related Compound A RS in methanol
Standard solution: 0.5 mg/mL of USP Irbesartan RS in methanol
Sample solution: 0.5 mg/mL of Irbesartan in methanol
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.0-mm × 25-cm; packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution
[ NOTE- The relative retention times for irbesartan related compound A and irbesartan are 0.8 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between irbesartan and irbesartan related compound A, System suitability solution
Relative standard deviation: NMT 1.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of irbesartan (C25H28N6O) in the portion of Irbesartan taken:
Result = (ru /rs ) × (Cs /Cu) × 100
ru = peak response of Irbesartan from the Sample solution
rs = peak response of Irbesartan from the Standard solution
Cs = concentration of USP Irbesartan RS in the Standard solution (mg/mL)
Cu = concentration of Irbesartan in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the anhydrous basis
IMPURITIES
• LIMIT OF AZIDE
Mobile phase: 0.1 N sodium hydroxide solution
Standard stock solution: 0.25 mg/mL of sodium azide in Mobile phase
Standard solution: 0.312 µg/mL of sodium azide in Mobile phase, from the Standard stock solution
Sample solution: 20 mg/mL of Irbesartan in Mobile phase
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: Conductimetric with a suitable background suppressor unit
Column: 4.0-mm × 25-cm; L31 packing
Flow rate: 1 mL/min
Injection volume: 200 µL
System suitability
Sample: Standard solution
Suitability requirements
Signal-to-noise ratio: NLT 10 for the azide peak
Analysis
Samples: Standard solution and Sample solution
Calculate the amount of azide, in ppm, in the portion of Irbesartan taken:
Result = (ru /rs) × (Cs/Cu) × (Mr1 /Mr2) × F
ru = peak area of azide from the Sample solution
rs = peak area of azide from the Standard solution
Cs = concentration of sodium azide in the Standard solution (µg/mL)
Cu = concentration of Irbesartan in the Sample solution (mg/mL)
Mr1 = molecular weight of azide, 42.02
Mr2 = molecular weight of sodium azide, 65.01
F = unit conversion factor, 1000
Acceptance criteria: NMT 10 ppm
• ORGANIC IMPURITIES
Buffer and Mobile phase: Prepare as directed in the Assay.
Standard solution: Use the System suitability solution, prepared as directed in the Assay.
Sample solution: 1 mg/mL of Irbesartan in methanol
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.0-mm × 25-cm; packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of irbesartan related compound A in the portion of Irbesartan taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of irbesartan related compound A from the Sample solution
rs = peak response of irbesartan related compound A from the Standard solution
Cs = concentration of USP Irbesartan Related Compound A RS in the Standard solution (mg/mL)
Cu = concentration of Irbesartan in the Sample solution (mg/mL)
Calculate the percentage of any other impurity in the portion of Irbesartan taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of any other impurity from the Sample solution
rs = peak response of Irbesartan from the Standard solution
Cs = concentration of USP Irbesartan RS in the Standard solution (mg/mL)
Cu = concentration of Irbesartan in the Sample solution (mg/mL)
Acceptance criteria
Irbesartan related compound A: NMT 0.2%
Any other impurity: NMT 0.1%
Total impurities: NMT 0.5%
SPECIFIC TESTS
• WATER DETERMINATION, Method I <921>: NMT 0.5%
ADDITIONAL REQUIREMENTS
• PACKAGING AND STORAGE: Preserve in tight containers, and store at a temperature below 30°.
• USP REFERENCE STANDARDS <11>
USP Irbesartan RS
USP Irbesartan Related Compound A RS
1-Pentanoylamino-cyclopentanecarboxylic acid [2′-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amide.
C25H30N6O 446.54